what can carbon bond with Carbon chemistry organic bond bonds angles four rules carbons hydrocarbon atom hydrogen molecule chemical only hybridized cardinal sp degrees each
When it comes to the fascinating world of organic chemistry, carbon plays a central role. The unique ability of carbon to form strong bonds with other atoms, especially carbon itself, allows for the creation of a diverse range of complex and interesting compounds.
Carbon to Carbon - Single, Double & Triple Bonds
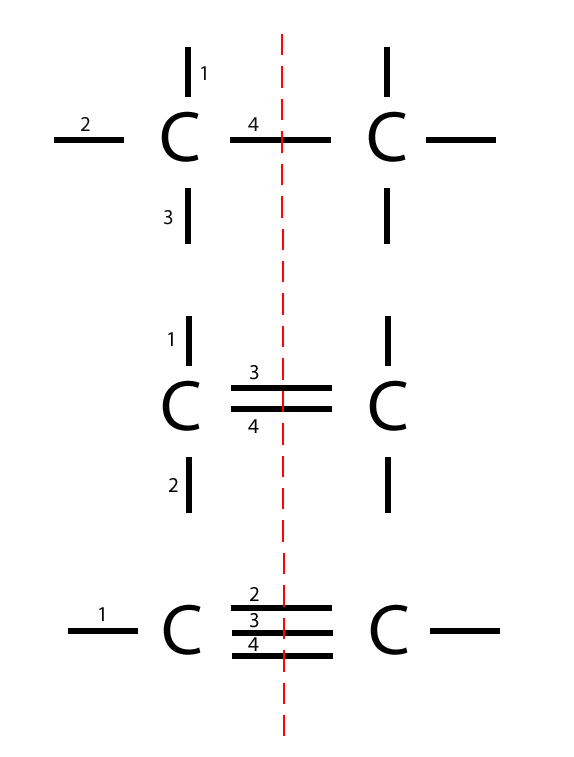 In organic chemistry, carbon-carbon bonds are of utmost importance and are classified into single, double, and triple bonds. These different types of bonds result from the sharing of electrons between carbon atoms. Let’s explore each type:
In organic chemistry, carbon-carbon bonds are of utmost importance and are classified into single, double, and triple bonds. These different types of bonds result from the sharing of electrons between carbon atoms. Let’s explore each type:
Single Bonds
A single bond between two carbon atoms is formed when they share one pair of electrons. This type of bonding is prevalent in many organic compounds and provides stability to the molecules. For example, in alkanes, every carbon atom is connected to four other atoms through single bonds, resulting in a linear or branched carbon skeleton.
Double Bonds
Double bonds occur when two carbon atoms share two pairs of electrons. This type of bonding is seen in compounds like alkenes. The presence of a double bond introduces rigidity into the carbon skeleton, affecting the shape and properties of the molecule. Additionally, double bonds are highly reactive and undergo various chemical reactions.
Triple Bonds
Triple bonds involve the sharing of three pairs of electrons between two carbon atoms. Compounds containing carbon-carbon triple bonds are known as alkynes. Due to their strong bonds, alkynes are often used in synthetic chemistry to create complex molecules.
5 Cardinal Rules of Carbon in Organic Chemistry
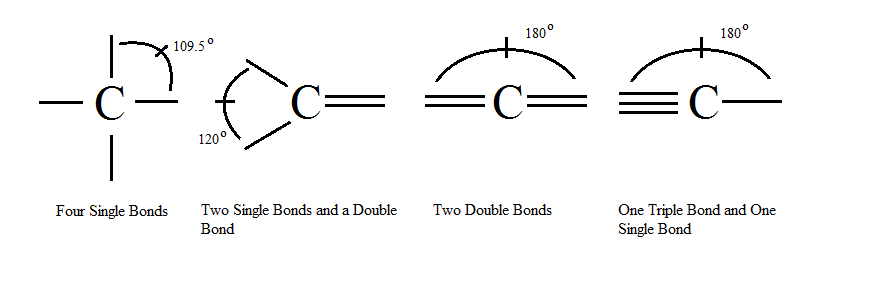 Understanding the rules that govern carbon in organic chemistry is essential to successfully navigating this vast subject. Here are five cardinal rules:
Understanding the rules that govern carbon in organic chemistry is essential to successfully navigating this vast subject. Here are five cardinal rules:
- Carbon has a valency of four, meaning it can form up to four chemical bonds. This enables it to have a wide diversity of compounds.
- Carbon has the unique ability to form stable bonds with other carbon atoms, leading to the formation of long carbon chains and complex structures.
- Carbon can also form bonds with other elements like hydrogen, oxygen, nitrogen, and halogens, leading to the creation of countless organic compounds found in nature.
- Carbon bonds are covalent, meaning electrons are shared between atoms to create stable molecules.
- Carbon-based compounds are the building blocks of life and are present in all living organisms and organic matter.
These cardinal rules form the foundation of organic chemistry and are crucial for understanding the behavior and properties of carbon compounds.
So, whether you’re studying organic chemistry or just curious about the world around you, take a moment to appreciate the significance of carbon bonding. From single bonds to complex structures, carbon’s versatility and ability to form strong bonds have shaped our understanding of the molecular world.
<h2>5 Cardinal Rules Of Carbon In Organic Chemistry - Organic Chemistry</h2>
<img alt="5 Cardinal Rules of Carbon in Organic Chemistry - Organic Chemistry" src="https://www.aceorganicchem.com/blog/wp-content/uploads/2017/08/four-carbon-bonds-1.png" width="100%" onerror="this.onerror=null;this.src='https://tse4.mm.bing.net/th?id=OIP.u1Ke_RE2VQMeggWZekJMAAHaCk&pid=15.1';">
<small>www.aceorganicchem.com</small>
<p>carbon chemistry organic bond bonds angles four rules carbons hydrocarbon atom hydrogen molecule chemical only hybridized cardinal sp degrees each</p>
<h2>Carbon Can Form Six Bond Molecule, Breaking The 4 Bond Limit | Latin</h2>
<img alt="Carbon Can Form Six Bond Molecule, Breaking The 4 Bond Limit | Latin" src="https://1622179098.rsc.cdn77.org/data/images/full/114240/carbon-bonding.png" width="100%" onerror="this.onerror=null;this.src='https://tse3.mm.bing.net/th?id=OIP.iUO0Y8covKE4Y4rVZrDykwHaEI&pid=15.1';">
<small>www.latinpost.com</small>
<p>form molecule breaking bonding</p>
<h2>Carbon To Carbon - Single, Double & Triple Bonds - Surfguppy</h2>
<img alt="Carbon to Carbon - Single, Double & Triple Bonds - Surfguppy" src="http://surfguppy.com/wp-content/uploads/2014/04/c-c-bonding.jpg" width="100%" onerror="this.onerror=null;this.src='https://tse4.mm.bing.net/th?id=OIP.btW0FJI5fcMpt4gPR2cATAHaJx&pid=15.1';">
<small>surfguppy.com</small>
<p>carbon bonding bonds triple single double example electrons atom navigation post chemistry</p>
<h2>What Is The Chemical Structure Of Carbon Chains And Rings?</h2>
<img alt="What is the chemical structure of carbon chains and rings?" src="http://www.biologyjunction.com/images/bondtypes.jpg" width="100%" onerror="this.onerror=null;this.src='https://tse4.mm.bing.net/th?id=OIP.ILBQl6vtUy6D1L9bpBEZEwHaGM&pid=15.1';">
<small>biology-forums.com</small>
<p>carbon chains bond structure biochemistry notes bonds double bi rings chemical</p>
<h2>Carbon To Carbon - Single, Double & Triple Bonds - Surfguppy</h2>
<img alt="Carbon to Carbon - Single, Double & Triple Bonds - Surfguppy" src="http://surfguppy.com/wp-content/uploads/2014/04/CARBON-TO-ARBON-BONDS.jpg" width="100%" onerror="this.onerror=null;this.src='https://tse4.mm.bing.net/th?id=OIP.k959V2k_EJ8lV0V-ygYA2wHaFd&pid=15.1';">
<small>surfguppy.com</small>
<p>carbon bonds triple double single drawing chemistry line middle made</p>